How can we trust our doctors and nurses that the therapies or drugs they prescribe may benefit us? Learn how clinical trials are used to demonstrate which treatments for diseases work and which don’t, and how one study in particular has already helped save over a million lives from COVID-19.
On the evening of 16 June 2020, millions of people tuned in to watch the Prime Minister, Boris Johnson, make a very special announcement. After months of despair and bad news, there was finally a ray of hope: for the first time, a treatment had been found that reduced deaths from COVID-19. What’s more, this drug (called dexamethasone) was cheap to produce and already used to treat other conditions, so it was known to be safe. It normally takes years to find effective treatments for diseases, so how was it possible that this discovery was made just three months after COVID-19 was declared a pandemic? The answer is the story of the remarkable RECOVERY Trial: a ground-breaking study that has smashed records, rewritten the rule book for health research and has been estimated to have saved millions of people worldwide.
A new disease with no treatment
When the World Health Organization declared a global pandemic on 11 March 2020, COVID-19 was a completely new, unknown disease. It would take at least several months to develop an effective coronavirus vaccine, or new treatments that specifically targeted COVID-19 symptoms. Consequently, many people suggested using existing drugs for other conditions to try and treat COVID-19, in case they had an effect. Some of these were recommended by major leaders (such as Donald Trump promoting hydroxychloroquine, a treatment for malaria), despite there being no evidence that they would actually have a benefit. Unless these drugs could be proven experimentally to reduce deaths from COVID-19 and improve patient outcomes, we would never know what actually worked and what didn’t.
Peter Horby, a Professor of Emerging Infectious Diseases at the Nuffield Department of Medicine, University of Oxford, knew this had happened during previous pandemics, including swine flu and the Ebola outbreak in Western Africa. Because potential treatments weren’t tested in large trials, no knowledge was gained on how to treat people affected by these diseases. Professor Horby joined forces with Professor Martin Landray, an expert in leading large-scale clinical trials at the Nuffield Department of Population Health, University of Oxford.

Professors Landry and Horby. The RECOVERY Trial’s Chief Investigators. Credit: OU Images/ John Cairns
Together they drew up a plan to launch a new study to test whether any existing treatments could be used to treat people with severe COVID-19, particularly whilst vaccines were being developed. This became the Randomised Evaluation of COVid-19 thERapY (RECOVERY) Trial.
How are new drug treatments tested?
Several different types of studies are used to test drug treatments (Box 1). The main two types are observational and intervention studies. In observational studies, the participants are not given any treatment and the investigators simply record what happens. By seeing which types of people develop a disease, they can find out what risk factors (such as alcohol or smoking) make this more likely.
Intervention studies, on the other hand, aim to find out whether a particular treatment may affect whether someone develops a disease or not. These include Randomised Clinical Trials (RCTs), considered to be the ‘gold standard’ for testing treatments. In an RCT, patients are divided into two groups. One of these acts as a control group, and receives a ‘dummy treatment’ (called a placebo) or no treatment. Participants in the experimental group, meanwhile, receive the treatment being tested. The participants are randomly allocated to a group, which means that the overall characteristics of the people in each group (such as age, weight and gender) are broadly similar. Consequently, if there is a difference in outcome between the two groups, this should be due to the effects of the treatment.
How to design a clinical trial during a global pandemic
The RECOVERY Trial is an RCT that is testing a range of different treatments (Box 2) in patients who are admitted to hospital with COVID-19. The treatments are compared against the standard hospital care that all patients receive. RECOVERY started with two experimental groups, to test Lopinavir-Ritonavir, normally used to treat HIV, and the steroid dexamethasone. But the trial was designed to be adaptive, so that more treatments could be added over time. Currently, 11 different treatments have been involved in the trial (Box 2), including newly-developed therapies designed to specifically target COVID-19. These treatments are divided into four main types to cover the different stages of disease: anti-viral, immunomodulatory, anti-thrombotic and anti-inflammatory.
Knowing that hospitals were already stretched to the limit, the trial leaders did several things to reduce the burden for doctors and nurses on the frontline. First, the recruitment process was made as easy as possible, using a simple form that takes minutes to complete. The participants are then randomly allocated by a computer to receive either one of the study treatments or standard hospital care (the control treatment). Second, the trial leaders focused on a few key outcomes: whether patients were still alive after 28 days, and whether their condition became so severe that they needed an oxygen mask or a mechanical ventilator to breathe. Instead of asking hospital staff to fill out more forms, the trial’s data scientists linked up to NHS databases, where hospital staff record this information as part of their normal duties (Figure 1). This meant they could track all the patients remotely using data from one source, without the hospital staff having to do any extra work. The trial’s researchers use unique reference numbers rather than names wherever possible, in order to maintain patient confidentiality. The participants give their consent for their data to be accessed by the research team when they sign up to the study.
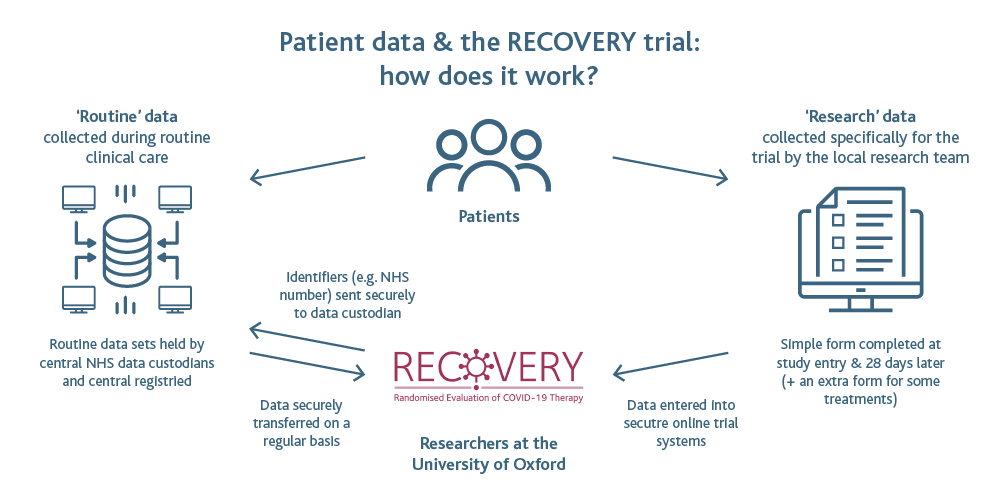
Figure 1: Diagram showing how the RECOVERY Trial combines information about patients recorded when they are recruited with routine data collected by hospital staff. This allows the trial’s research team to track each patient remotely, without causing extra work for doctors and nurses. Image credit: Understanding Patient Data.
It normally takes months for a new clinical trial to be approved but because the situation was so urgent, the RECOVERY Trial launched on 19 March 2020 – only nine days after the idea was first written down. This was helped by the UK’s regulatory and research authorities and the Research Ethics Committee fast-tracking the process to approve the trial, and the UK Government providing support and funding.
What makes the RECOVERY Trial special?
RECOVERY wasn’t the only COVID-19 clinical trial taking place of course. But what set it apart was its sheer scale. The trial leaders aimed to recruit at least 2,000 patients to test each treatment – a number that takes most clinical trials months or years to achieve. But they knew this was the only way to generate reliable, robust data. Most other clinical trials investigating COVID-19 across the world involved far fewer patients, often less than a hundred. This increases the likelihood that the results arose by chance, rather than because of the treatment being investigated. Consequently, these small studies often had conflicting results, leaving doctors no wiser about whether a treatment actually works against COVID-19. The RECOVERY Trial aimed to settle these debates by involving thousands of patients. This would give it strong statistical power, so that even moderate increases in patient survival could be detected. Because of its large scale, the results from RECOVERY are widely regarded as the most reliable evidence available on what works against COVID-19.
In order to recruit so many patients, the trial partnered with the NHS to launch simultaneously in 176 hospitals across the UK. Fortunately, this happened just before the first coronavirus wave struck the UK, so the hospitals were ready to recruit as many patients admitted with COVID-19 as possible. In just two months, over 10,000 patients had been recruited (Figure 2). Unusually for a clinical trial, RECOVERY was fully inclusive and open to all patients with COVID-19, even children and pregnant women.
COVID-19 affects everyone and the urgency and scale of the pandemic meant there was an immediate need for knowledge about treatments that would be suitable for all groups of people. Most of the treatments being investigated have previously been used in pregnancy for other medical conditions without safety concerns being raised. However, pregnant women did not receive baricitinib or colchicine as they may be harmful in pregnancy or when breast-feeding. See www.recoverytrial.net/for-site-staff
The data from the trial were reviewed every two weeks by an independent data monitoring committee (DMC), so that the public could be sure the results would be trustworthy. Because the trial was recruiting patients so quickly (Figure 2), by June the DMC realised that there was a significant difference in the outcomes of patients who received dexamethasone and those who received normal hospital care.

Figure 2: Graph showing the daily patient recruitment rate to The RECOVERY Trial over time. Image credit: RECOVERY Trial
For patients who had the most severe form of COVID-19 and needed a mechanical ventilator to breathe, the death rate was a third lower in the group who received dexamethasone, compared with the control group. Around 41% of the patients in the control group died, whilst only 29% in the dexamethasone group died. For patients who needed an oxygen mask (but not a ventilator), dexamethasone reduced deaths by a fifth (21.5% vs. 25.0%). This meant that one death would be prevented by giving dexamethasone to around eight ventilated patients or around 25 patients requiring oxygen alone.
The trial’s researchers were delighted – it was the moment they (and the whole world) had been waiting for. Because this was literally life-saving news, it was important to make the result known to the public as quickly as possible. Once the data had been double-and triple-checked, the discovery was announced to the UK Government, the Department of Health and Social Care, journalists and healthcare providers. The result was reported across the world in thousands of television programmes, radio broadcasts, online news articles and newspapers. Within a few hours, NHS guidelines were updated so that every patient hospitalised with COVID-19 would automatically receive dexamethasone. Hospitals in other countries soon did the same. Since dexamethasone is cheap, easy to administer because it is a tablet and widely available, it could be used immediately in all kinds of settings. It has been estimated that between June 2020 and March 2021, dexamethasone saved around 22,000 lives in the UK, and approximately a million worldwide.
The second wave
The dexamethasone result was an incredible achievement, but the trial continued to investigate more treatments (Box 2), even when COVID-19 cases dropped significantly during the summer (Figure 2). In winter 2020/2021, new highly-infectious coronavirus strains caused a second wave to sweep across Europe, and hospital admissions with COVID-19 skyrocketed. At the peak of this, RECOVERY was recruiting 500 patients a day! Thanks to RECOVERY’s work so far, all these patients were receiving better treatment, since dexamethasone was now part of their standard hospital care. But in February 2021, RECOVERY found that combining dexamethasone with tocilizumab, a drug normally used to treat arthritis, had an even greater impact. Together, these two drugs reduced deaths by about one third for patients using an oxygen mask and nearly one half for those who needed mechanical ventilation. Patients who received tocilizumab were also less likely to deteriorate so that they needed intensive care, and they had a shorter average stay in hospital – including RECOVERY Trial participant Wendy Coleman (Box 3).
Both dexamethasone and tocilizumab are immunomodulatory treatments. They work by blocking the body’s own immune system signalling pathways that can cause excess inflammation in patients with severe COVID-19. But another way to combat COVID-19 is to use anti-viral treatments that attack the coronavirus itself. The RECOVERY trial was able to test a new, experimental antiviral treatment designed to specifically block coronavirus particles from entering human cells. It contains two antibodies (in a combination called casirivimab with imdevimab) that both recognise and bind to the coronavirus spike protein. Normally, the immune system produces antibodies when exposed to the coronavirus (either the virus itself or a vaccine). But in some people – including the elderly, or those taking immunocompromising drugs such as cancer treatments– this may not happen (or might take longer to happen), making them significantly more vulnerable to developing severe COVID-19. On June 16 2021 – exactly a year after the dexamethasone result- the RECOVERY trial discovered that the experimental antibodies were effective in hospital patients who had failed to produce coronavirus antibodies of their own. For these people, the drug reduced the risk of death from COVID-19: instead of 30% of such people dying without this treatment, only 24% did. Excitingly, this offers an effective treatment for those who can’t take a COVID-19 vaccination, or for whom a vaccine hasn’t been effective.
In addition, the trial has also found six other treatments that weren’t effective against COVID-19 (Box 2). These include drugs developed for other conditions and convalescent plasma: a product made from blood donated by patients who have recovered from COVID-19. Although these results were disappointing, this knowledge is still important. Healthcare providers can now prioritise their resources, rather than spend money and time giving patients treatments that don’t work (even if some people still claim they do!).
What’s next for the RECOVERY Trial?
RECOVERY is continuing to investigate several treatments in the UK (Box 2). Because each participant’s NHS records contain a wide range of data (such as their GP records and medical history) and are continually updated over time, the research team will be able to analyse the long-term effects of COVID-19, such as later lung problems or kidney disease. This year, RECOVERY also expanded internationally to Indonesia, Nepal and Vietnam, in order to explore which treatments are most suitable for developing countries.
RECOVERY has shown that clinical trials can be done quickly and at scale, when they are easy to introduce into healthcare systems, and don’t place additional burdens on hospital staff. Their strategy of linking up with existing patient records in the NHS could be used to reduce costs and increase efficiency for all types of clinical trial in the future. This is the goal of NHS DigiTrials, which helps researchers to access databases of health information. Ultimately, this could accelerate the discovery of treatments for all sorts of chronic diseases. Even when COVID-19 has passed, we will still need health research. Cardiovascular diseases, for instance, kill around 17.9 million people worldwide each year whilst COVID-19 caused approximately 1.8 million deaths in 2020. It’s clear that the RECOVERY Trial could still be having an impact many years from now.
Additional information
Glossary
Antibodies – Also known as immunoglobulins, these are Y-shaped proteins that bind to foreign invaders (such as bacteria and viruses), helping the immune system to destroy them.
Anti-inflammatory – These block inflammation, an immune system response against invaders that involves releasing chemicals and proteins (including antibodies), and can result in redness and swelling.
Anti-thrombotic – A drug that stops the formation of blood clots. In severe COVID-19, blood clots can form in the patient’s lungs.
Anti-viral – A treatment that prevents a virus from infecting the body, for instance by stopping a virus from binding to receptors that allow it to gain entry to cells.
Immunomodulatory – A drug which changes the response of the body’s immune system, either increasing it (immunostimulants) or decreasing it (immunosuppressives). In COVID-19, an excessive immune response can cause hyperinflammation.
Statistical power – The ability of a study to detect an effect that is really there. In health research, it can be described as the probability of avoiding making the conclusion that a treatment has no effect, when it actually does.
Steroids – Also known as corticosteroids, these are anti-inflammatory medicines that are used to treat various illnesses.
Find out more
Please note that the below links open in a new tab
RECOVERY Trial website: Here you will find all the information about RECOVERY including the study’s design, news updates, results data and video interviews with the trial’s leaders.
Dexamethasone announcement: Watch Professor Sir Peter Horby announce the dexamethasone result during a 10 Downing Street press conference:
NHS DigiTrials: Learn how the NHS is making more data available to support clinical trial research.
Inside Health: RECOVERY Trial: This special edition of BBC Radio Four’s health programme celebrates the RECOVERY Trial’s impact a year after its launch. Hear from the trial leaders, besides a doctor on the front line and a patient who took part.
Understanding Patient Data blog: This article gives more detail on how routine patient data is used by the RECOVERY Trial.
NIHR website: The National Institute of Health Research (NIHR) is one of the main funders of clinical research in the UK. Their website is a treasure trove of information about different types of trials. It also lists the studies currently taking place and describes how the public can be involved in research.
Nuffield Department of Population Health (NDPH)
About the author
Caroline’s job is to communicate important (but often complicated) health research so that the public, policy makers and healthcare providers can understand it. Before joining Oxford University, she was a policy officer for the UK Research Councils. After her degree in Cell Biology at Durham University, she completed a PhD at the University of Sheffield, studying parasitic plants.
Download PDF
If you wish to save, or print, this article please use this pdf version »


