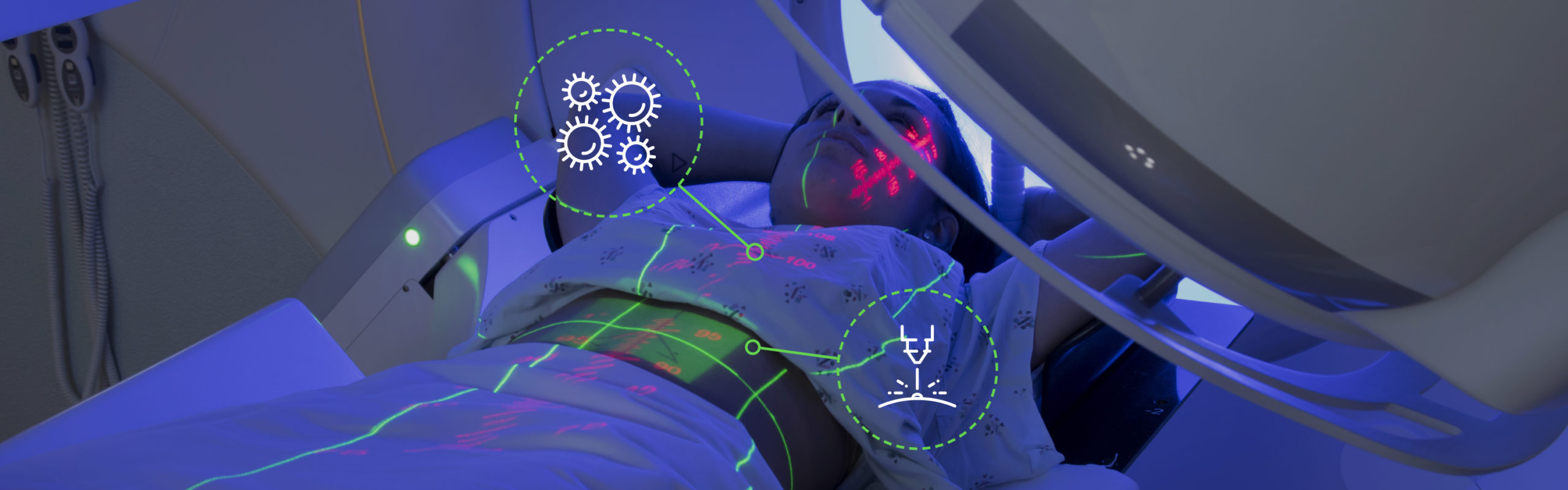
Radiotherapy is one of the main cancer treatments available and has evolved quickly since it was discovered. This article aims to showcase some of the new innovations that are being used by departments in the UK.
 ^ Image 1 – A linear accelerator
^ Image 1 – A linear acceleratorRadiotherapy has been a widely utilised cancer treatment for over 100 years and in that time it has undergone many changes. When a patient receives radiotherapy, linear accelerators (linacs – Image 1) are used to deliver high energy x-ray photons to a targeted area of the body. These photons are absorbed by both cancerous cells and healthy tissue in the body, so it is important that radiotherapy can be given to maximise tumour kill whilst minimising normal tissue damage, this is known as the therapeutic ratio. Once the cancer cells have been targeted enough times they are not able to recover and will go through cell death, whereas healthy tissue will recover over a period of time. Radiotherapy dose is measured in Gray (abbreviated to Gy and named after Louis Harold Gray, a British physicist involved in the measurement of x-rays). Generally, most patients will receive between 2 and 3 Gy per day. The number of treatments a patient will receive varies between one and thirty seven with it usually taking place on consecutive days Monday – Friday, with a break over the weekend.
Modern linear accelerators allow the treatment of cancer to be accurate to within 2 millimetres.

Tailor-made treatments
Every patient coming through for treatment has an individualised plan that has been produced by medical physicists, based on an initial computed tomography (CT) scan of their body that helps the oncologist pinpoint exactly where the tumour or residual cancer cells are located to provide the highest possible dose to the cancer cells whilst giving the lowest dose to other areas of the body. The initial CT scan of the patient is used to plan their treatment and manufacturers of linacs have now been able to add on-board x-ray imaging to the treatment machines. This enables us to take a scan on each of their treatments to make sure we are targeting the correct area whilst avoiding the organs at risk. Within the head of the linac there are multi-leaf collimators (MLC’s). These are beam-limiting devices that are able to shape and vary the radiotherapy beam to accurately target the treatment area (MLC’s – Image 2). These are adjusted for each individual patient based on the area being treated and which other organs are nearby that we don’t want to treat such as the heart, lungs, kidneys and eyes. Many of these organs have maximum doses they can be given before they will not recover and because of this, it is important for the physicists and oncologists to take time to produce a plan that reduces the dose to these areas as much as possible.
 ^ Image 2 – A multi leaf collimator
^ Image 2 – A multi leaf collimator
New advances = less treatment time
There has been a lot of research recently into whether we can give higher doses over less time because some tumour types respond better to larger doses. With improved imaging and MLC’s we now have the technology in place to allow this. Recent research into a radiotherapy technique called stereotactic ablative body radiotherapy (SABR) has allowed us to start patients on treatment pathways where they can receive up to 18Gy per treatment to certain areas, such as lung tumours. This is possible due to the fact that we can get clear CT images of the patient whilst on the treatment couch, utilising the on board imaging that modern linacs have allowing treatments to be accurate to within 2 millimetres. Previously patients would either have not had treatment or would have been treated with larger radiation fields to allow for the decreased accuracy. Older MLC’s were not capable of adjusting to a smaller area, and without excellent imaging the radiographers couldn’t be sure enough that they were in the right place. This has meant that patients who may not have been able to have surgery for their cancer, for example because their risk is too high due to medical complications, can now have these new types of treatment instead. It has a high chance of controlling the patient’s cancer and means they would have to come in for fewer appointments due to the higher dose they will receive per treatment.
Radiographers preparing for the future
Radiographers will never stop learning as more innovations such as the one discussed above are brought into practice. We may train in radiotherapy at university however we expand our roles to enable us to undertake new types of imaging such as CT and ultrasound so we are better equipped to give the best care and treatments to all of our patients. It’s an exciting and rewarding career to be in, especially when there are opportunities to keep learning and gaining knowledge in different areas.
CT – computed tomography, commonly known as a CT scanner this takes cross sectional images with an x-ray tube mounted on a ring gantry so it rotates around the patient gathering images in a spiral.
Gray (or Gy) – the unit that radiotherapy dose is measured in.
Medical Physicist – they train in medical physics and perform all the checks on our linacs and scanners to ensure they are accurate and able to deliver treatment safely. They also plan each patients’ individual treatment alongside oncologists.
MLC – multi-leaf collimators, mounted in the head of a linac these thin leaves of tungsten shape the radiotherapy beam.
Oncologist – a consultant who specialises in oncology (cancer) care.
Useful links
radiographycareers.co.uk – good resource to see which area of radiography would suit best!)
www.sor.org – Society of Radiographers
An Aardman Animations video aimed at our younger patients about the radiotherapy pathway.
Cancer Research UK – more information on SABR
Learning resource
We have created learning notes to assist students and educators to further investigate the topics covered in this article. You can download the learning resource here »