As you have probably noticed there are already lots of battery powered and battery hybrid cars and vans on the roads. There are though, a few problems with batteries. If they could be used for longer, reused and recycled and made from less rare materials, their environmental and monetary cost to us would be less.
Petrol and diesel are convenient forms of fuel because we know how to safely store and burn them. We are familiar with how these fuels work with an internal combustion engine. We know how far a vehicle will travel on a single tank and how much it will cost. On top of that, petrol and diesel cars are reasonably priced. They do however have major downsides, they are a huge source of carbon dioxide and their fuel source is not sustainable. As a result, people are investing in electric vehicles to help reduce pollution and impact on the environment. But electric cars are expensive, and they cannot travel as far as their polluting cousins.
What is more, they take much longer to charge than simply putting petrol in the tank. A typical electric car with a 60kWh battery can take up to 8 hours to fully charge from empty. Filling up at the petrol station takes less than five minutes. The batteries in an electric car are not yet able to take you on a comparable journey with a similar sized petrol or diesel counterpart either in distance or the time it takes. So electric vehicles are not without their own downsides, but these can be addressed. Make them more accessible in price and improve the performance of their batteries.
Manufacturers are researching how reliable, sustainable and cheaper batteries will hasten people to switch from traditional cars much more quickly. A first step in this transition is to help us to understand the limitations and potential of lithium-ion batteries. A second step is to make electric batteries less wasteful, more cost effective and to generate a use, reuse and recycle methodology.
Understanding the battery
Batteries for cars are usually made from hundreds of individual cells. The materials used in lithium-ion car batteries have lithium metal oxides on one side and graphite on the other, and lithium ions shuttle between them. The metal oxides contain nickel, manganese, and cobalt. The most expensive metal is cobalt, which is only found in large amounts in the Democratic Republic of the Congo, where mining practices cause major health and safety problems. But using fewer cobalt means using more nickel, and high nickel content materials are less stable.
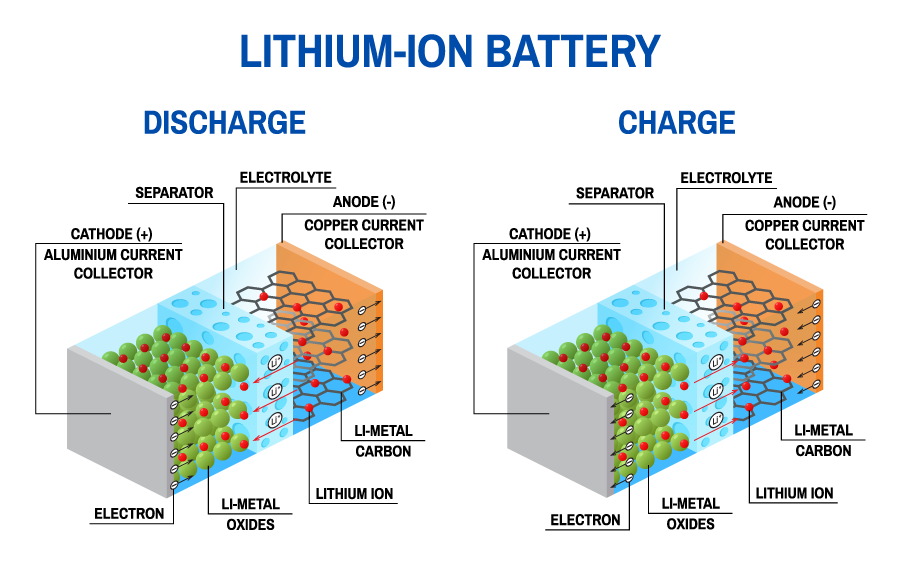
Common lithium-ion battery cell demonstrating charge and discharge
Some of the research happening in this field is to understand why. One of the issues is that the materials are stuck in an electrolyte which is an organic (carbon based) liquid and a corrosive salt. The salt breaks down when it encounters water and forms a very strong acid. This acid reacts with the metals in the lithium metal oxides, meaning there is less ability to store lithium. This is further complicated as the metals dissolved in the electrolyte cause problems on the other electrode.
The other side of a battery is made of graphite, like that found in an ordinary pencil. This stores lithium in between its layers. But lithium is very reactive in graphite, and at the edges of the graphite it reacts with the electrolyte forming a protective sludge on the surface when it charges up, using up a little bit of the lithium. Some additives can be added so this sludge stays flexible as the lithium comes and goes from the graphite. This stops too much lithium being wasted by forming the sludge. The battery then keeps the same amount of lithium to shuttle back and forth, so the amount of charge the battery has stays the same. But those dissolved metals might disrupt the sludge layer, meaning more lithium is wasted forming the sludge and there is less to carry charge back and forth. This means every time the battery is charged; it will last for less time before it needs recharging.
Another solution might be to change the shape of the particles of material or even give them a coat. By changing the shape, the reactions that happen on the surface change. This is a bit like trying to scale a mountain by different routes. A steeper route is likely to be difficult and likewise, some sizes of particles react more than others. A small, steep mountain with a higher surface to volume ratio compared to a large mountain. Large particles have less reactive edges than small particles, but the insides of them can be very tricky for the lithium ions to reach, making their charging and discharging slow. Imagine you are waiting for your car to finish charging at the electricity stations, how quick do you want it to be?
Exploring efficiency
Related to that problem, have you noticed that your phone does not actually know how much charge your battery has left? It can take hours to go from 30% to 20% charge, and then suddenly die. The difficulty is in measuring how much of the batteries ability to charge and discharge remains available. New measurements are being designed to record how the battery has been treated throughout its life. This is being achieved by using maths, computer models and machine learning, to assess all the data from batteries constantly being charged and discharged in research labs. This will enable huge amounts of real-life data to be stored in car computers about the battery’s performance. The computers in electric cars already decide which cells, of the hundreds they have, to use. Enabling them to protect the overworked cells from getting too tired, too hot and from causing irreversible reactions.
It is not enough to describe how good a battery is by how many years old it is, some cells age more quickly than others. Being able to measure how a cell has been treated is important to know if it should be retired, or if it can be reused with other cells in another battery.
Reuse and recycle
Once a battery cannot be used any more, we need to think about recycling. At present, they are put through a giant shredder, like a paper shredder. Then the parts can be melted down and the metals recovered. But there are only a few of these in the world and environmentally it is not advisable to ship decaying and corrosive batteries long distances. It might be a better idea to reuse the existing battery by scraping materials off the layers by machine, and just put more lithium in chemically, rather than making the materials from scratch again. Perhaps there is potential to use the batteries as a storage system for the electric grid, which in theory is feasible, if not yet in practice.
By making the materials more stable, better protected and the right size, they will last longer, especially if carefully packed together so that the optimum temperature is maintained. By better understanding the charging and discharging, we can monitor the health of the battery. Helping to identify when they require replacement. Enabling us to extend the batteries life and reduce the need for non-sustainable materials.
Looking to the future
Our current lithium-ion batteries can achieve some of the fastest 0-60 mph speeds for a car. In fact, the fastest cars are often hybrid systems of electric motors and engines. Currently an electric car is the fastest four-door car ever built. This is impressive power. But lithium-ion batteries priority should be to deliver longer driving distances between charging and have longer lives. By developing the battery to be more cost effective with reusable cells it will help drive down the cost of production, reduce waste and lessen the impact on the environment. Helping to make electric cars more accessible and desirable.
Download PDF
If you wish to save, or print, this article please use this pdf version »
Learning resource
We have created learning notes to assist students and educators to further investigate the topics covered in this article. You can download the learning resource here »


