At any one time, between one fifth and one quarter of the world’s population has a fungal infection. These infections of skin, hair and nails are difficult to treat. Modelling these infections provides a better understanding of how disease occurs, but there’s a problem… these fungi only infect humans.
Superficial fungal infections affect 20-25% of the world population at any one point in time. These are non-lethal infections of the skin, nails and hair, which are better known as ringworm, athlete’s foot, eczema and dandruff. Skin infections are mainly caused by fungi called dermatophytes which produce enzymes that allow them to break down keratin – a protein – found in skin, nails and hair. The most common dermatophyte fungus causing athlete’s foot and ringworm is Trichophyton rubrum. Dandruff and some eczema is caused by another type of fungus, called Malassezia. This fungus uses oils from our skin to grow and divide as they cannot make their own.
Although fungal skin infections are incredibly common, we still do not completely understand how and when infection occurs, how infection progresses and why we get flaky skin. Understanding these things will allow better treatments to be developed, as these infections remain difficult to treat or require treatment over a long time.
So why don’t we know what happens during infection?
Normally to investigate how infections start and progress we model these infections. This means that we infect a host with the fungus and watch how infection progresses. Often this is carried out in animals, such as mice. However, we cannot do this with dermatophytes that infect humans. Why not? This is because dermatophytes such as Trichophyton rubrum are anthropophilic (human preferring) and those that infect animals are zoophilic (preferring animals other than humans). If we infect mice with a human dermatophyte, we do not see a long-term infection with development of flaky skin, instead we see lots of inflammation and the infection is rapidly cleared. Obviously, this is not the same as what we see in humans who get athlete’s foot or ringworm. This means that mice are not a good model of these infections in people and results found in mice will not reflect what happens in people. So how can we model these infections to better understand what happens during infection?
In a 3Rs (Replacement, Reduction and Refinement) approach, we have replaced the use of animals and have developed a human skin model to investigate what happens when fungi infect human skin. Our research uses excess human skin left over from surgical operations, where patients have said that the excess skin can be used for research – informed consent. In the research lab, the skin is cleaned to remove any contaminating bacteria and fungi. It is then cut into small squares, which are infected with fungi. Using tissue culture medium and conditions, we can keep the skin alive in the lab for up to 14 days. This allows us to closely watch how infection starts and what happens to the skin over this time.
After infecting human skin with the athlete’s foot fungus, Trichophyton rubrum, the fungus changes from yeast cells to a network of long hyphal cells. We can see signs of infection on the skin at day 3, with scaly/flaky skin lesions seen on day 10. These lesions are similar to those seen in patients with ringworm or athlete’s foot. Using scanning electron microscopy, we showed that fungal hyphae were found in the skin and that the upper skin layer became detached from the layer below (becoming smooth rather than the rough surface seen in uninfected skin), creating flaky skin.
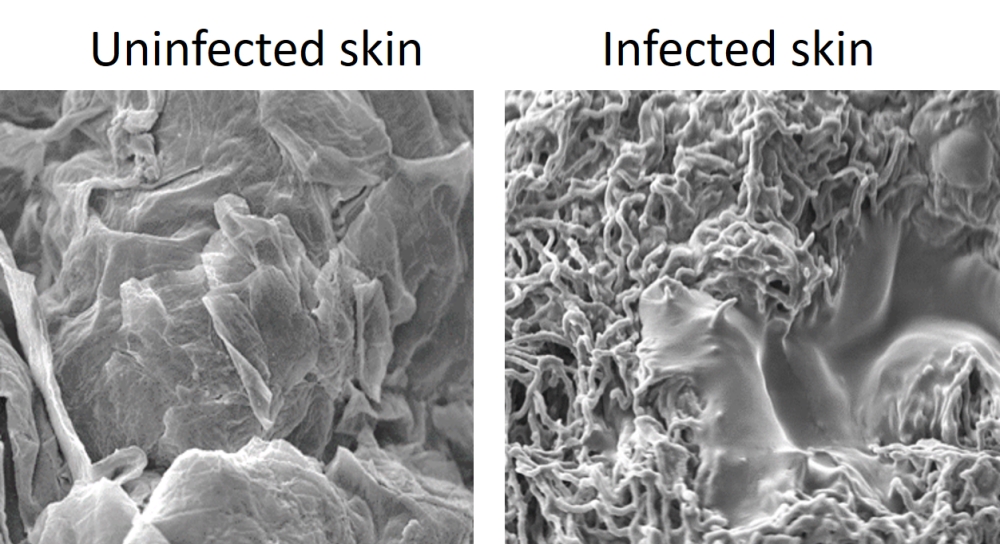
Image captured by Dr Dora Corzo Leon and the Microscopy and Histology Core Facility at the University of Aberdeen.
By measuring gene expression in fungal cells and in skin cells during infection, we obtain information about the proteins and signalling molecules produced during infection and how they affect the host response to infection. Identifying crucial steps in the infection process will identify targets for new treatments for these infections.
We have used the same skin model to investigate how Malassezia behaves when it infected oily skin. To do this, Malassezia cells are used to infect normal skin or skin with added oil. Without oil, the fungus remains as yeast cells, but with addition of oil the fungus grows as normal yeasts and as elongated cells. Using proteomics to measure and identify fungal proteins in the skin, we showed that fungal cells grown on oily skin secreted more allergen proteins than fungi grown on normal skin. Further investigation of the role of each of these allergens in eczema will be carried out in the future. Host skin immune responses were also measured, with Anti-Microbial Peptides (AMPs) increased in Malassezia infected skin but reduced when the infection occurred on oily skin. This suggests that Malassezia grown with oil can affect AMP expression, reducing the immune response against the fungus. Again, identifying crucial steps in the infection process is essential for finding more effective treatments for these infections.
Together, these experiments demonstrate that using animal infection models is not always the best way to investigate infections, particularly if the infecting microorganism does not normally infect the animal used. Use of animal models for these infections would have provided information on a rapid host immune response, leading to clearance of the fungus. Instead, using real human skin in lab experiments, a longer lasting infection occurred, with alterations to the skin surface, leading to production of flaky skin. This clearly demonstrates that researchers need to carefully consider whether an animal model is appropriate for their research or whether alternatives, such as use of human tissue, could replace the use of animals. After all… the best model for any human infection or disease must be human!
Download PDF
If you wish to save, or print, this article please use this pdf version »
Learning resource
We have created learning notes to assist students and educators to further investigate the topics covered in this article. You can download the learning resource here »