Discover the inside world of medical devices from plasters to MRI scanners, how they are designed, exciting future developments, the scientists, engineers, and clinical staff involved, and the great lengths engineers go to, to prove designs are safe.
Did your nan get a new hip recently, or has your dad got an artificial knee? Do you know someone who has a “stent”, to keep their arteries open? Have you had an x-ray or MRI scan? We talk about “medical devices” all the time, but often don’t think about how they are designed and made. A medical device is anything that is used to diagnose, monitor, or treat disease or disability. You can probably think of dozens of medical devices yourself: pacemakers, syringes, medical gloves, thermometers, plasters, heart monitors, scalpels, and insulin injectors – to name just a few!
If you stop to consider a few things, you start to realise just how much work there is behind medical devices. To get an x-ray image of bones, the patient must be exposed to x-ray radiation. We know the x-ray radiation is damaging if you are exposed for too long – but exactly how much radiation is unsafe? Artificial knees are often made of titanium alloy or cobalt-chrome alloy (amongst other options) – how do we know it’s safe to leave that material in the body for over 10 years?
And what about the people who contribute to the research, design, and manufacturing of medical devices? It’s a highly cross-functional process, with input from clinicians (doctors, surgeons, nurses), research scientists (biologists, chemists, materials science), and then realised by engineers (of many varieties – design, manufacture, mechanical, electrical).
Lots of my examples in this article will use orthopaedic medical devices (joint replacements) because that is the area that I have experience with – I used to work on the design and manufacture of knee and hip replacements.
How to design and make a medical device
As with any design, it starts with an idea. The idea could be from a doctor/surgeon who wants to solve a problem for one of their patients, or from an engineer. It could even be from someone who doesn’t know anything about science or engineering but has an idea for how to solve a problem. That’s what design is all about – solving problems.
If the idea is new enough – for example, if you need to develop a new material that is stronger or lighter than those available – you might need to do some research. Research is conducted by all manner of scientists either within companies’ research departments or at universities. Depending on the problem you are trying to solve, you might need to work with chemists, biologists, materials scientists, physicists, or any other area of science you can think of really!
When you are confident that the technology you need is available (we call this feasibility – the design can be made), it’s time to develop the design. We usually start by coming up with an initial design, then making a prototype. The prototype can be used to prove that your idea works, but it’s also useful to understand what you need to improve, and how easy it was to make. You could have four or five iterations of designs and prototypes before the design is finalised, whilst issues are ironed out. It’s important to be resilient in this stage.
The final step is to make as many of the devices as you need to sell. Manufacturing can be anything from a couple of people in a workshop to enormous factories with thousands of staff, depending on what you are making and how many devices you want to make. The important thing about manufacturing medical devices is that it is controlled. This means that you have a specification that the manufactured device must meet (usually engineering drawings). The devices are made in the same way, using the same equipment and staff each time, so that they always meet the specification (we call this a validated process). Finally, the manufactured devices are inspected against the specification to check the quality. Any devices that don’t meet the specification cannot leave the factory.
There are lots of international standards and laws that set out requirements for medical devices. Laws are specific to each country (or region, such as the EU), and generally set out what a company must do to be allowed to sell medical devices there – the laws make sure the public is kept safe. International standards are assembled by technical experts and are documents that describe the best knowledge or methods we have (as a global community) in a particular area. Some of them are quite general (for example, the international standard BS EN ISO 13485:2016 sets out how a company should be set up to maintain the quality of medical devices), whereas others are very specific to certain device types or aspects of their manufacture (for example, the international standard BS EN ISO 11137-1:2015 identifies how processes used to sterilise medical devices with gamma radiation should be validated).
An example in detail – Total Knee Replacement
The knee joint is like a complex hinge. The main motion of the joint is bending or straightening your leg, where the condyles of the femur (upper leg bone) slide against the tibia (lower leg bone), to form the hinge. There is also some evidence that the femur moves across the tibia in some activities (called anterior glide) and rotates a little (called medial pivot). The patella (kneecap) protects the front of the knee, but also gives additional leverage to the quadriceps tendon, allowing the quadriceps muscle to act more efficiently. The image below shows the anatomy of the knee.
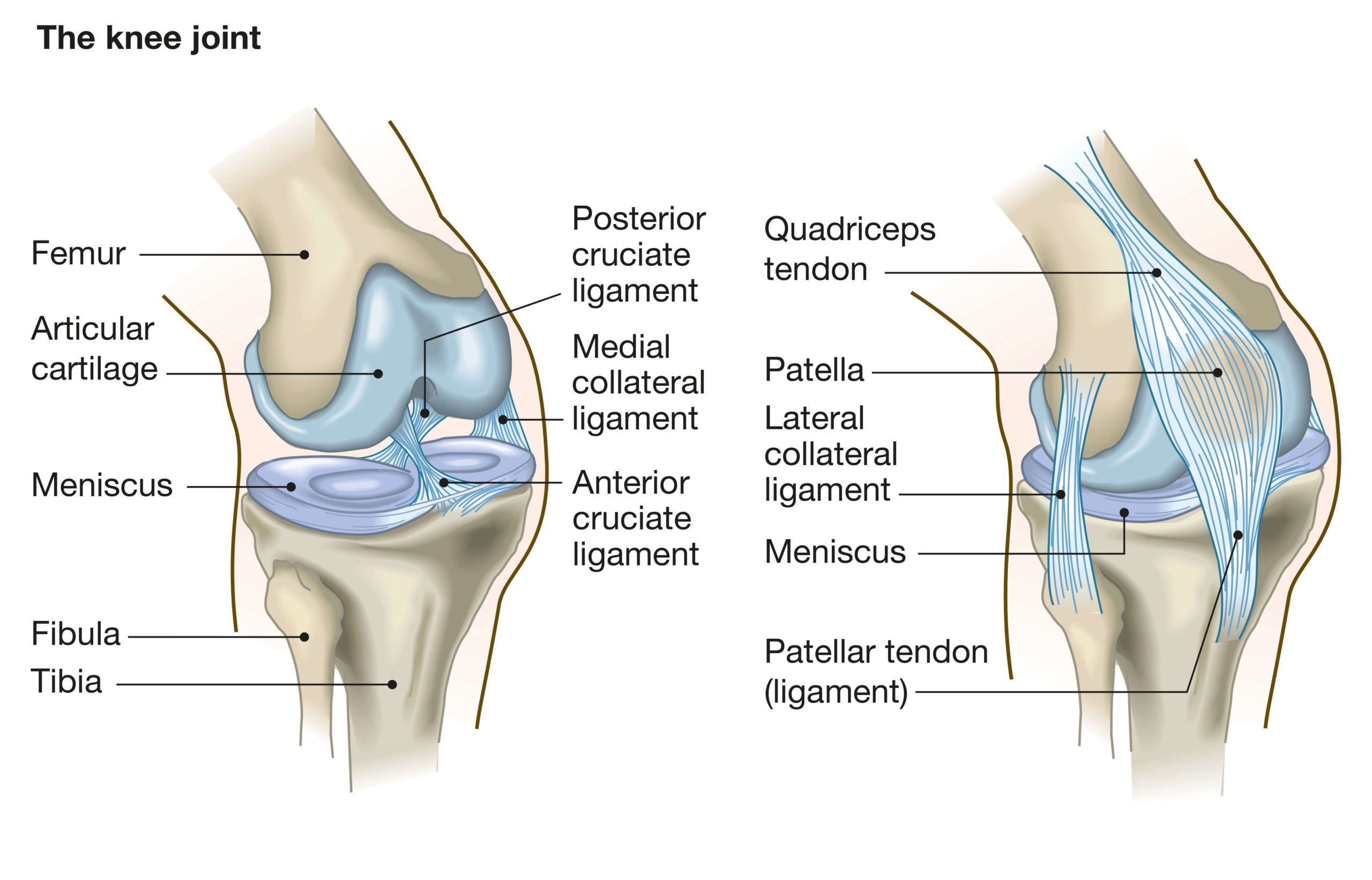
Image: Anatomy of the knee joint
The bearing surfaces of the tibia and femur are covered with cartilage (called the meniscus). This naturally wears down over time, resulting in thin cartilage that can be painful during walking or other activities; this is called osteo-arthritis, which is the main reason for total knee replacement (or TKA – total knee arthroplasty). The goal of TKA in this scenario is to reduce the pain the patient experiences and allow them to move freely. There can also be other reasons for TKA, such as bone fractures in the joint region that may reduce the range of motion of the knee joint.
In the case of treating osteo-arthritis, the purpose of the prosthetic knee components is to provide a new bearing surface, which will ideally last the lifetime of the patient. Some further requirements of the design are that it osseo-integrates well (forms a strong connection with the bone), and withstands the forces of walking, running, and other physical activities. The components must also be made of materials that are safe for long term implantation.
Components of a total knee replacement
In a standard TKA surgery, the goal is to remove as little bone as possible – the natural structures of the body have the advantage of millions of years of evolution, and we want to leave as much as possible. The image below shows the typical components used in a knee replacement system – there are many different designs available, this is a generalised picture. A small amount of bone is removed from the top of the tibia and replaced with a metal component. A small amount of bone is also removed from the end of the femur, in a particular shape (using specially designed cutting guides) and replaced with a metal component. In between, and secured to the tibial component, is a plastic spacer. The new bearing surface is formed by the metal femoral component against the plastic spacer. The plastic spacer can be replaced if it wears out (which is expected to be many years later). Sometimes, a small amount of bone is removed from the patella and replaced with a plastic component – this depends on the level of wear on the natural patella bone.
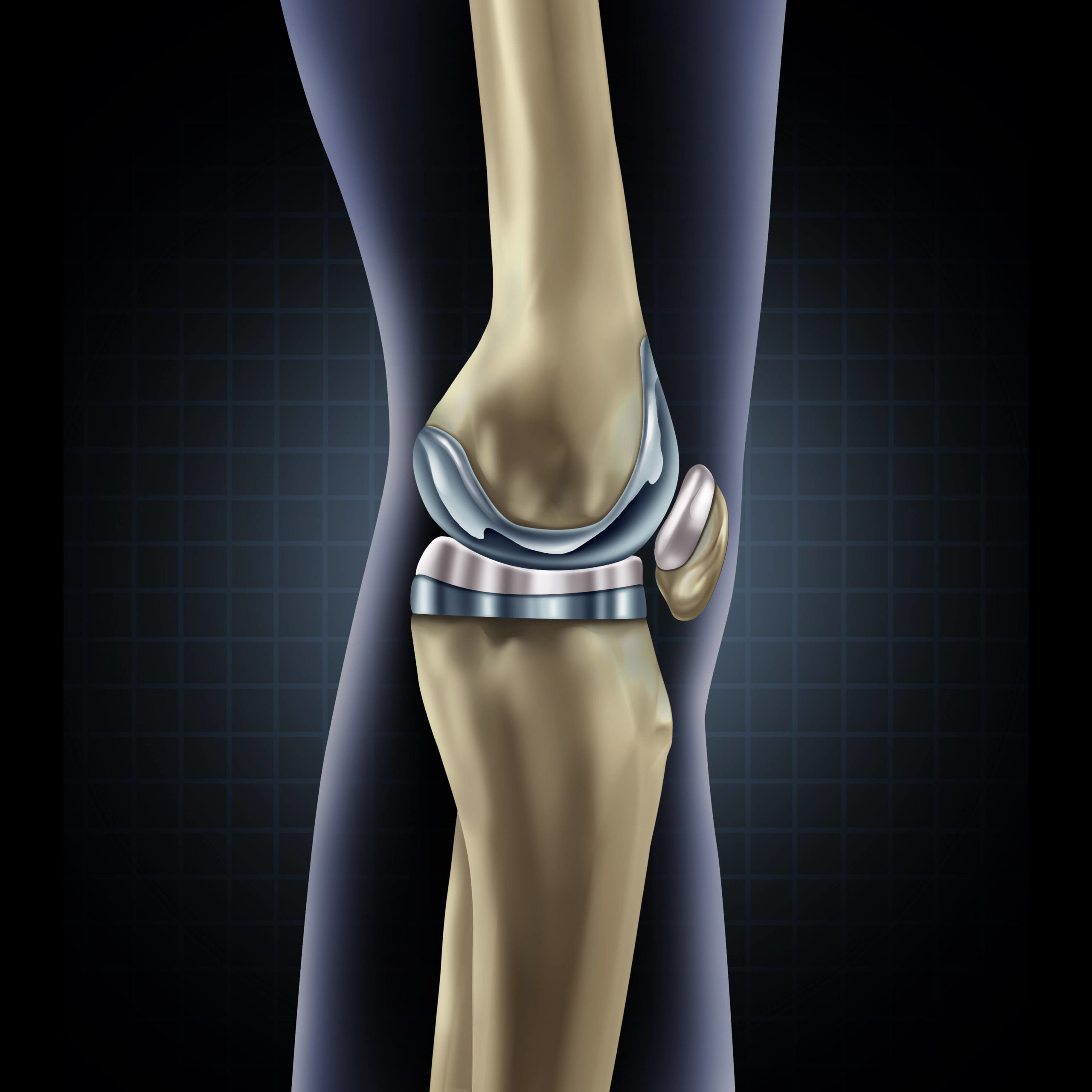
Image: A typical total knee replacement
Design considerations
I mentioned that the material needs to be safe for implantation (biocompatible). The metal parts are usually either a Cobalt-Chromium alloy or a Titanium alloy (particular “medical grade” versions), as these have a history of performing well as implantable materials. The plastic parts are usually medical grade polyethylenes such as ultra-high molecular weight polyethylene or highly cross-linked polyethylene. Some companies have infused vitamin E into these polymers, as it reduces the oxidation rate of the polyethylene, which is the main way this material “ages” (gradually weakens).
The shape of the condyles of the femoral component is an important design factor. Engineers base their designs on natural knees (using images from x-rays or CT scans), and which previous designs have worked well. This has developed a lot over the last 20-30 years, and the latest designs are now very effective in producing a similar motion to the natural knee.
It’s important that the metal components osseointegrate well. Traditionally, these components are bonded to the bone using something called “bone cement”, which is an acrylic polymer that is mixed during the surgery and sets between the implant and the bone. Many modern implants have been designed not to require bone cement, to achieve a more natural fixation. These implants have advanced surfaces. The metal surface itself is roughened, to give the bone more surfaces to adhere to. The surfaces intended to touch the bone are coated with specialist coatings containing hydroxyapatite, which is one of the main elements of natural bone scaffold substances (containing calcium) and promotes osseointegration between the implant and the natural bone. The latest developments are to 3D print metals so that they are porous; the idea is that the bone can grow into the porous metal, forming an even stronger connection. This strong connection prevents loosening, which is one of the main ways in which TKA components can fail.
One of the important ways to test a total knee design is a fatigue test. When a force is applied to a component millions of times, the metal (or plastic) can fail at a much lower force than would be required to cause failure in a single application of force. Think about how to break a paper clip – it doesn’t break the first time you bend it, but bend it 10 or 20 times, and it will break. A similar concept applies to engineering components, including medical devices, but usually isn’t an issue until millions of loading cycles. To test a total knee design, the components are loaded into a test rig that can apply a force 30 times a second, so that a lifetime’s worth of walking and running can be tested in a matter of days.
Is it safe?
The motivation behind all the activities that engineers (and other STEM professionals) perform whilst developing a medical device is to answer the question “is it safe to use?” This section is intended to give you some insight into the lengths we go to, to answer that question.
Science & engineering
An important aspect to consider is biological safety. There are important international standards in this area that clearly define how to investigate the biological safety of a medical device (18 of them in fact – the ISO 10993 series of standards). Some of the considerations for an implant, for example, are:
- Cytotoxicity (being toxic to cells – causing poor cell function or cell death)
- Systemic toxicity (being toxic to the body overall, or particular organs)
- Genotoxicity (being damaging to genetic information)
- Carcinogenicity (causing cancer)
The engineers designing medical devices usually work with biological specialists to ensure that the right biological risks are identified, the right testing is performed, and to determine whether the test results show that the device is safe for its purpose.
Another key aspect is the mechanical performance of a medical device – particularly important for weight-bearing implants such as hip and knee replacements. For the total knee replacement example, we mentioned the need for fatigue testing. But there are other kinds of mechanical testing that can be required – such as static testing (applying an increasing force until failure), torsional testing (applying an increasing twisting force until failure) or bending the component until failure.
Active medical devices – those with an electrical aspect – also need to be tested to ensure the safety of their electrical systems. For example, it would be very important to test the battery in a pacemaker, to ensure it lasts long enough before it needs to be replaced (many years). A rather different example would be that for an MRI machine, which produces a strong magnetic field; the electronics controlling the machine still function despite the magnetic field (which could interfere with the electronic systems).
Clinical data
All the lab testing evidence helps to demonstrate that a medical device is safe, but some of the most important data is clinical data. This is evidence from use of the device in its real-life application.
The highest risk devices, and those with brand-new technologies that haven’t been seen before, usually need a clinical investigation. This means that, based on the lab testing, the manufacturer will be authorised to make a small number of devices to use in a clinical trial. These devices will be used by doctors/clinicians, within a well-defined clinical study, to determine aspects of the safety and performance that could not be demonstrated by lab testing. As you can imagine, these studies have strict ethical and safety requirements.
All other devices need a clinical evaluation report to justify the safety and performance in the clinical application. In this report, the manufacturer will usually compare their new device to existing devices that are already available. For example, in relation to a new knee replacement, there are numerous designs that have been in use for years, with many scientific papers and studies published on their safety and performance. By making comparisons between the new design and previous designs, the manufacturer can make an argument for the predicted safety and performance of the new design.
The manufacturer must also make a commitment to monitoring the safety and performance of their new device, using various methods. Some of these methods are quite straight forward – things like monitoring complaints for any trends (for example, if there were lots of complaints of compromised sterile packaging). For some devices, the manufacturer will need to commit to a follow-up study in which they will work directly with clinicians and patients to measure the performance of the device. This might involve patients having regular follow up appointments with their doctor or surgeon to gather data on the device; for example, for a knee replacement, completing a questionnaire on the health of the knee.
Risk
To check that all possibilities have been considered, engineers conduct a risk analysis, often called a failure mode effect analysis. We gather a team of designers (and others) and have a brainstorm about all the ways the device could break or otherwise harm the patient. For each possible failure, we give it scores for the probability that it might happen, and the severity of the effect if it does happen. This is a way to take the subjectivity out of assessing the risks around a particular medical device. By using scoring methods, it’s a more objective assessment, and takes human bias out of the process. If any of the scores are too high, we can take action to reduce either the probability that the failure will happen, or the severity of the effect if the failure does happen.
For example, a risk for a plaster or wound dressing may be that it gets wet when the patient washes. The probability of this is high, as people generally wash regularly! The severity of the effect depends on what the wound dressing is covering. If it is a plaster covering a small cut, the severity will be low, as the cut is unlikely to be negatively affected by being washed, and the plaster can easily be replaced. Now consider a wound dressing for a recent surgical site; this is much more critical, as it needs to control moisture around the wound but also aid in preventing infection. If water compromises the dressing, it could compromise the infection control – this is a more severe affect. Therefore, we might choose to take an action to reduce the chance that the wound dressing can be affected by water, for example using a more appropriate adhesive.
Notified Body assessment
After all the measures that the designers take to ensure the device is safe, they can’t just sell start to sell it; they need to ensure that it meets the laws of the country they intend to sell it in, and sometimes also need a certificate to be able to sell it. My description below is in relation to the rules in the European Union, as this is one of the most advanced systems in the world for regulating medical devices. The UK has an almost identical system since Brexit.
For the lowest risk devices (like a simple surgical scalpel), the company must check that they meet the legal requirements, then make a legal declaration that they have met the requirements. For higher risk devices (from relatively simple devices such as a dental filling to more complex devices such as a pacemaker), the manufacturer must apply to a notified body for assessment against the legal requirements. A notified body is a company that has been assessed to have staff with expertise in medical devices, and granted a special designation allowing them to perform the assessments. This ensures that the safety of all devices (except the simplest) is checked independently of the manufacturer, by experts. If the device meets the requirements, the notified body issues a certificate to the manufacturer, allowing them to sell the device in the European Union. Similar systems operate in most countries. Notified bodies usually just check that the devices comply with the law (and international standards), but the checks also help to detect fraud.
Final thoughts
I hope this article has sparked some interest in thinking about all the medical devices that we take for granted. You have seen the great variety of STEM professionals that are involved in the research, design, manufacture, testing and use of medical devices. Perhaps you are interested now too; if you are, read my other article about routes into the industry. Also watch out for my article in a later issue about some interesting technologies that may be used in medical devices of the future.
Download PDF
If you wish to save, or print, this article please use this pdf version »


